
3 was the so-called charged liquid-drop model. The earliest description of a nucleus that could successfully characterize the binding energy curve of Fig. See also: Mass Nuclear binding energy Nucleon The transformation of a very heavy nucleus into two major fragments is the fission process, and the difference in binding energy (or, equivalently, mass) between the initial nucleus and the final system provides fission’s large energy release. The small binding energies of the lightest nuclei do not favor breaking into many small pieces. Consequently, it is possible that a nucleus of greater mass ( A > 56) could find it energetically favorable to convert to a more stable system by breaking into lighter fragments nearer to this peak. 3, can be seen to occur around mass A = 56. A larger binding energy per nucleon implies a more tightly bound and thus a more stable nucleus. 3, has the interesting behavior of a rapid increase followed by a slow decline. Careful examination of the variation of the binding energy per nucleon ( BE/ A) with total mass, as in the curve shown in Fig. The average binding energy is approximately 8 MeV per added nucleon for stable nuclei, and decreases slowly for exotic isotopes of a given element. The binding energy that is released during the formation of a nucleus is what would have to be supplied to decompose the nucleus back into its individual components. This mass difference is emitted in the form of energy and is called the binding energy of that nucleus.
#NUCLEAR FISSION EQUATION FREE#
When an ensemble of free nucleons (protons and neutrons) come together, the nucleus that is formed has less mass than the sum of the masses of the individual free nucleons. For example, one of many possible fission reactions these scientists may have explored is written as:
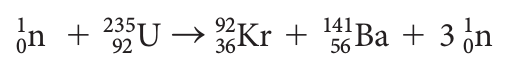
They explained that the uranium nuclei following neutron capture are highly unstable and split in a process that Frisch coined as “fission,” in analogy to the biological process of cell division. However, it was Meitner and Austrian-born British physicist Otto Frisch, now supported by the undisputed results of Hahn and Strassmann, who provided the correct understanding of the counterintuitive experimental findings in 1939. Ida Noddack, another German chemist who had worked with Fermi, had earlier proposed that lighter elements could be formed by this type of bombardment. Their work further revealed that the energy released in the bombardment was orders of magnitude greater than any previously known decay. Through meticulous radiochemical techniques, Hahn and Strassmann confirmed in 1939 that barium was indeed present among the products, along with many other intermediate-mass nuclei, following the bombardment of uranium by neutrons. See also: Atomic nucleus Coulomb excitation Energy Isotope Nuclear physics PhotonĪlthough this undertaking eventually proved successful, it initially yielded confusing chemical results by seemingly also producing a lighter element, barium ( Z = 56), in very high yields. The development of nuclear physics and the fission process during the twentieth century has played an important role in the technical sector, for instance, with regard to electricity production and medical therapies, as well as affected cultural development and political decision making.

More broadly, fission results from disruption of the delicate balance between the attractive nuclear force and the repulsive Coulomb force within a large nucleus and is driven by the fact that nuclear binding energy is maximized for medium-mass nuclei. Fission is a naturally occurring spontaneous decay process of heavy isotopes and can also be induced by the absorption of particles, such as neutrons, protons, or photons, under appropriate conditions ( Fig. The extreme deformation of a very large nucleus followed by splitting of the nucleus into lighter nuclei, predominantly two lighter fragments, in conjunction with a large energy release. Delayed neutron emission by fragments allows control of the chain reactions.

Prompt neutron emission during fission provides the capability for a chain reaction of fission events. This probabilistic nature of fission implies that each fission event and its resulting mass and energy distributions are different. Isotopes have an independent fission yield, which is a probability that they will be produced in any given fission event. The liquid-drop model of the nucleus provides an effective qualitative and quantitative explanation of fission.Ī typical fission event releases a total of around 200 million electronvolts (MeV) of energy. The fission process is governed principally by nuclear binding energy and the competition between the attractive nuclear force and the repulsive Coulomb force. A nuclear fission event is the splitting of one nucleus into two or more lighter nuclei fragments.


 0 kommentar(er)
0 kommentar(er)
